Results
Patients
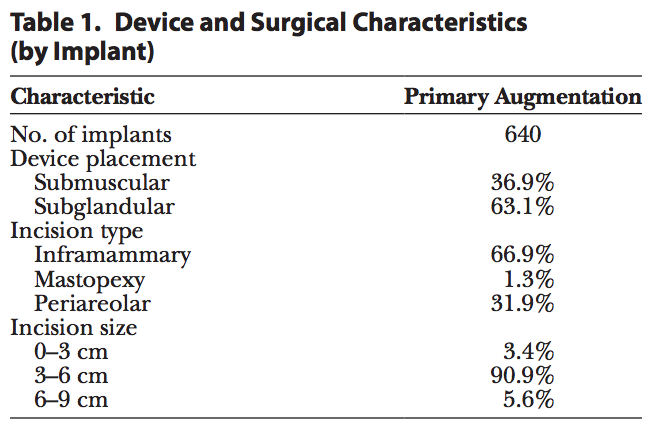
Analysis was performed on 321 primary augmentation patients. The reported median patient age at the time of enrollment was 36 years. The majority of patients (60%) reported having an annual household income of over $60,000, and 85% had at least some college education. The median height and weight at enrollment were 5′5′′ (SD, 2.7) and 128 lbs (SD, 21.0), respectively, and median body mass index was 21 (SD, 3.1). All implants included in this analysis (Table 1) were Sientra HSC+ implants. An inframammary incision site was most commonly used (67%), and the majority of implants were placed in the subglandular position (63%), with an incision size ranging from 3 to 6 cm (91%).
Safety Experience
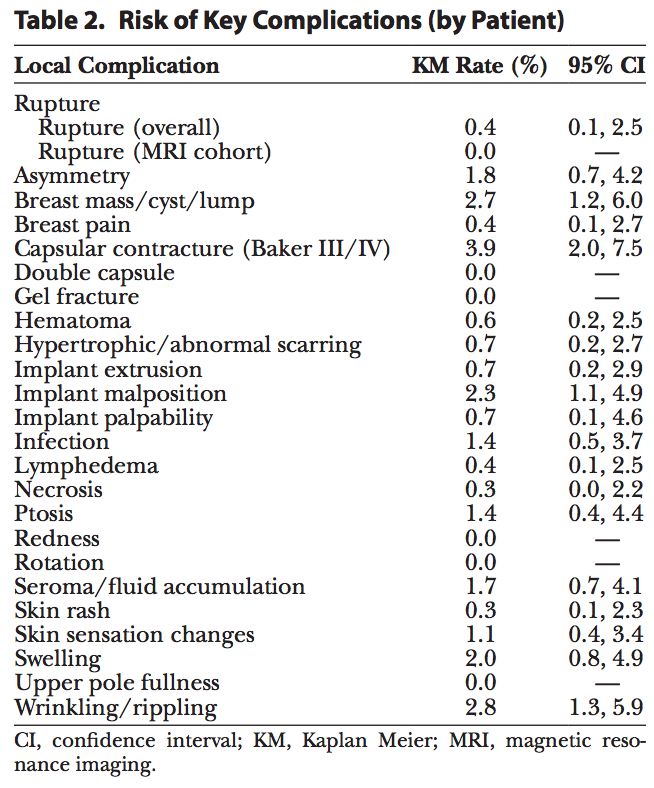
Table 2 summarizes the complication rates for all reported complications through 5 years post-surgery. Within the key complications, the overall risk of rupture for primary augmentation patients implanted with Sientra shaped implants was 0.4%, the risk of capsular contracture was 3.9%, and the risk of infection was 1.4% (Table 2). Other complications occurring with a risk over 2.0% were breast mass/lump/cyst, implant malposition, and wrinkling/rippling.
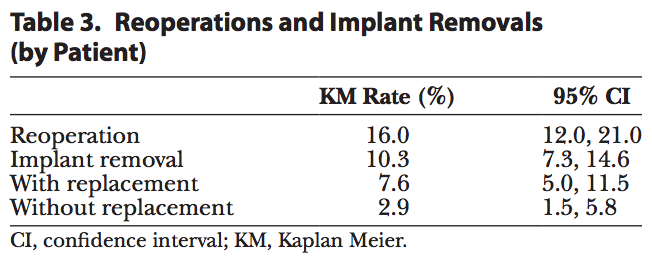
Table 3 reports the rates of reoperation and removal. Forty-four patients underwent reoperation after implantation, with two thirds (68%) of reoperations occurring before 2 years in vivo (30 of 44 patients). The risk of reoperation was 16.0% by patient and consistent with or lower than that with the other approved shaped implants.[4,5] Of the 44 patients who underwent a reoperation, 21 patients were explanted with replacement and 8 patients were explanted without having implants replaced. The risk of explantation (with or without replacement) was reported to be 10.3%. The 2 most common reasons for explantation were style/size change (46%) and capsular contracture (12%); all other reasons occurred at less than 10%.
Effectiveness
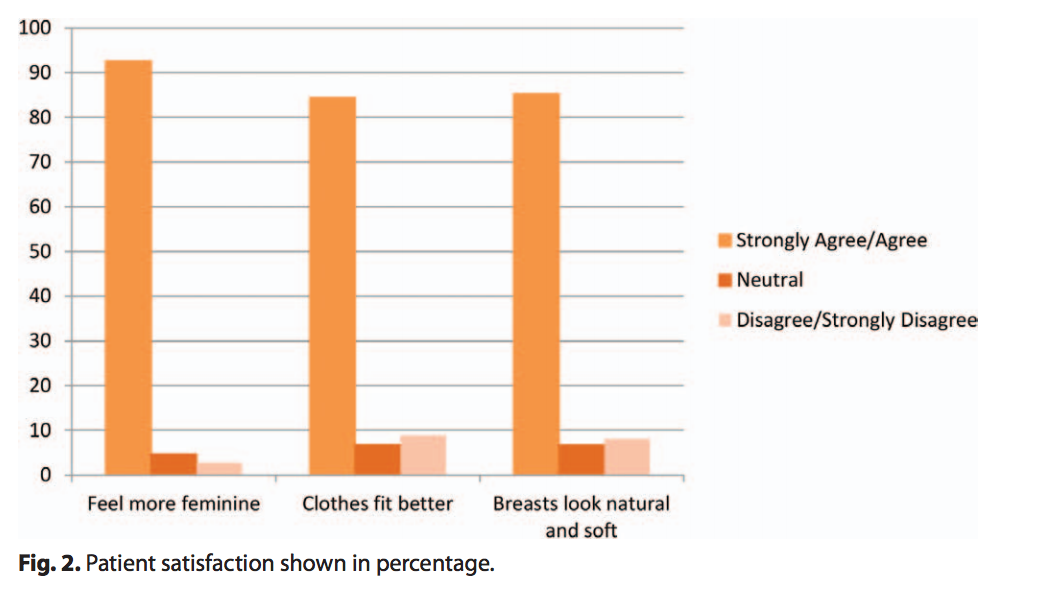
At the completion of the implant surgery, 100% of surgeons reported satisfaction with the surgical results for all procedures. Over 87% of patients increased their bra cup size by at least one size. Additionally, the majority of patients felt that their implants made them feel more feminine, look natural and soft, and made their clothes fit better (Fig. 2).
Single-Surgeon Methods
To explore a single surgeon’s experience, a separate frequency analysis was performed on surgical characteristics (incision site and implant placement) and incidence of complications for a series of 108 primary and revision augmentation cases by one of the authors (M.R.S.). This author did not participate in any of the shaped core studies; therefore, he had no prior experience using shaped implants. Patients implanted with Sientra’s shaped implants between June 2012 and October 2013 were included in this analysis. The data reported are through up to 16 months of follow-up time, with a mean follow-up of 9 months.
Single-Surgeon Results
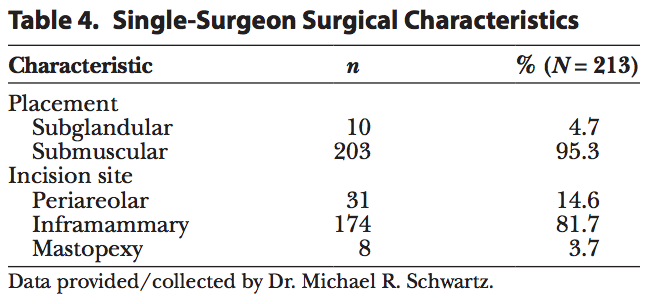
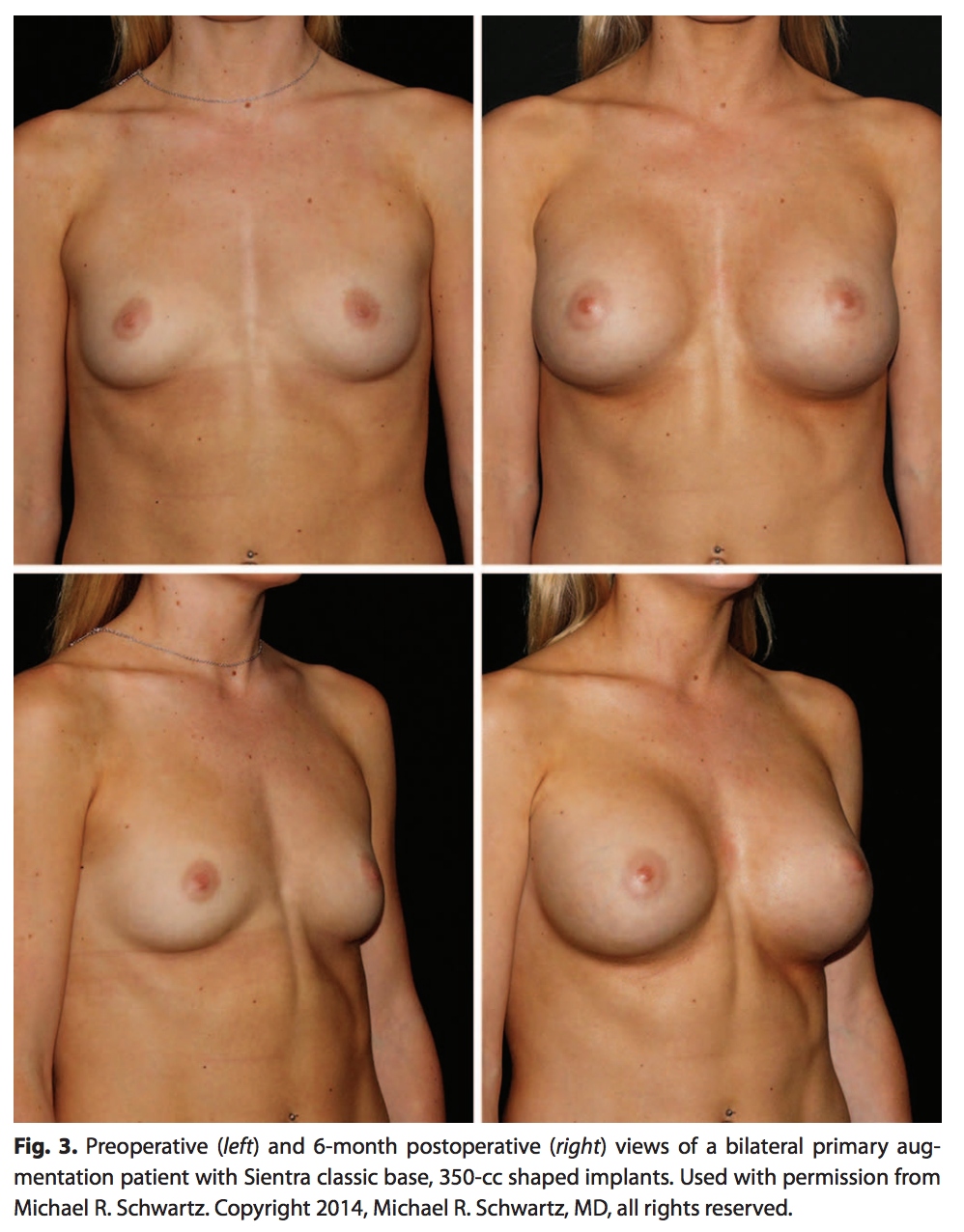
A single surgeon’s experience with 108 patients and 213 shaped implants was analyzed. For this population, 95% of the implants were submuscularly placed and the remaining 5% were placed in the subglandular position (Table 4). The majority of the implants (82%) were placed via an inframammary incision (Table 4). Photographic results are shown in Figure 3.
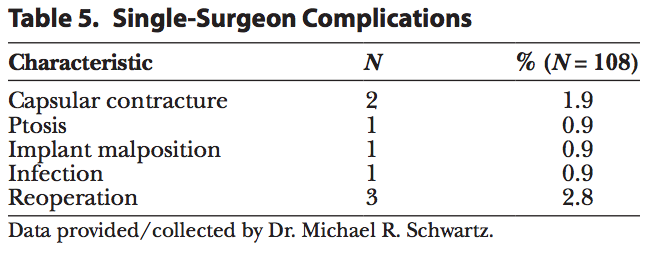
Of the 108 patients implanted, 4 experienced a complication (3.7%) (Table 5) and 3 underwent a reoperation (2.8%) for ptosis, infection, and capsular contracture through 16 months. The single-surgeon complications presented in Table 5 were capsular contracture (1.9%), implant malposition, infection, and ptosis (0.9% each).
Clinical Experience
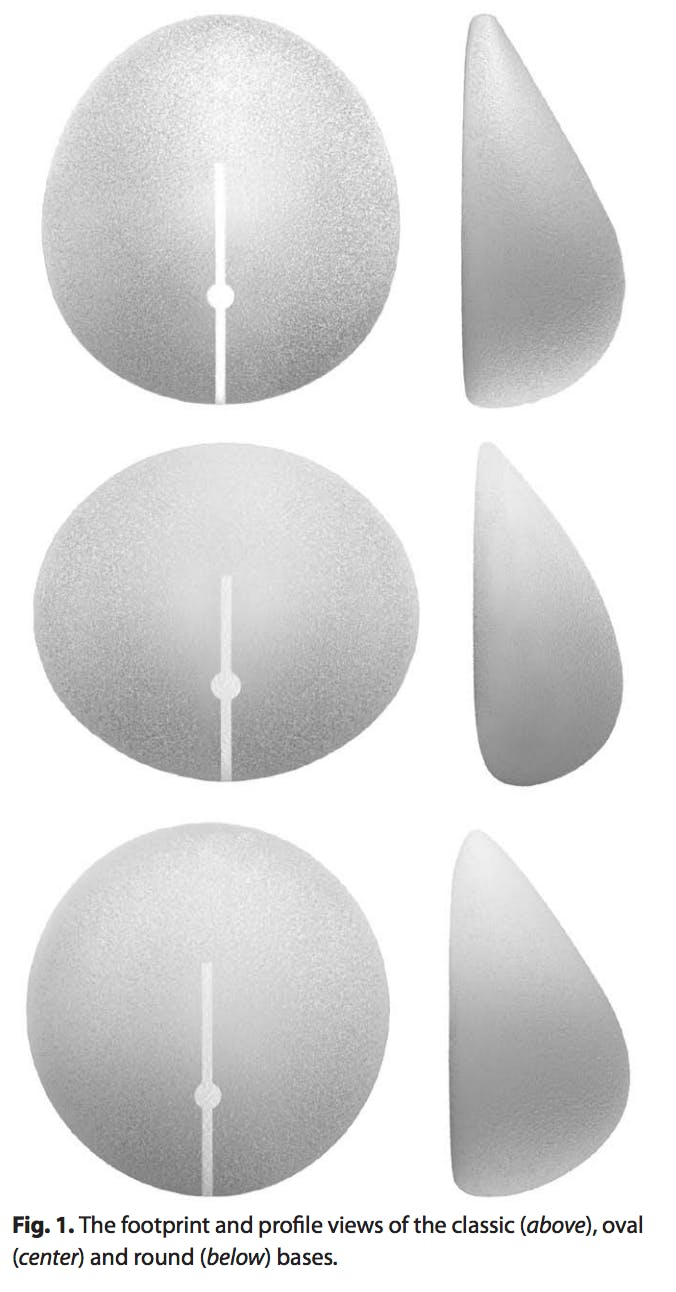
There are 3 styles of shaped implants, each with a unique base: oval, round, and classic. Each base fulfills an individualized need to address the wide variety of body and breast sizes and shapes and allows the surgeon the ability to provide a shape to women who were not previously able to achieve an acceptable outcome with round implants.
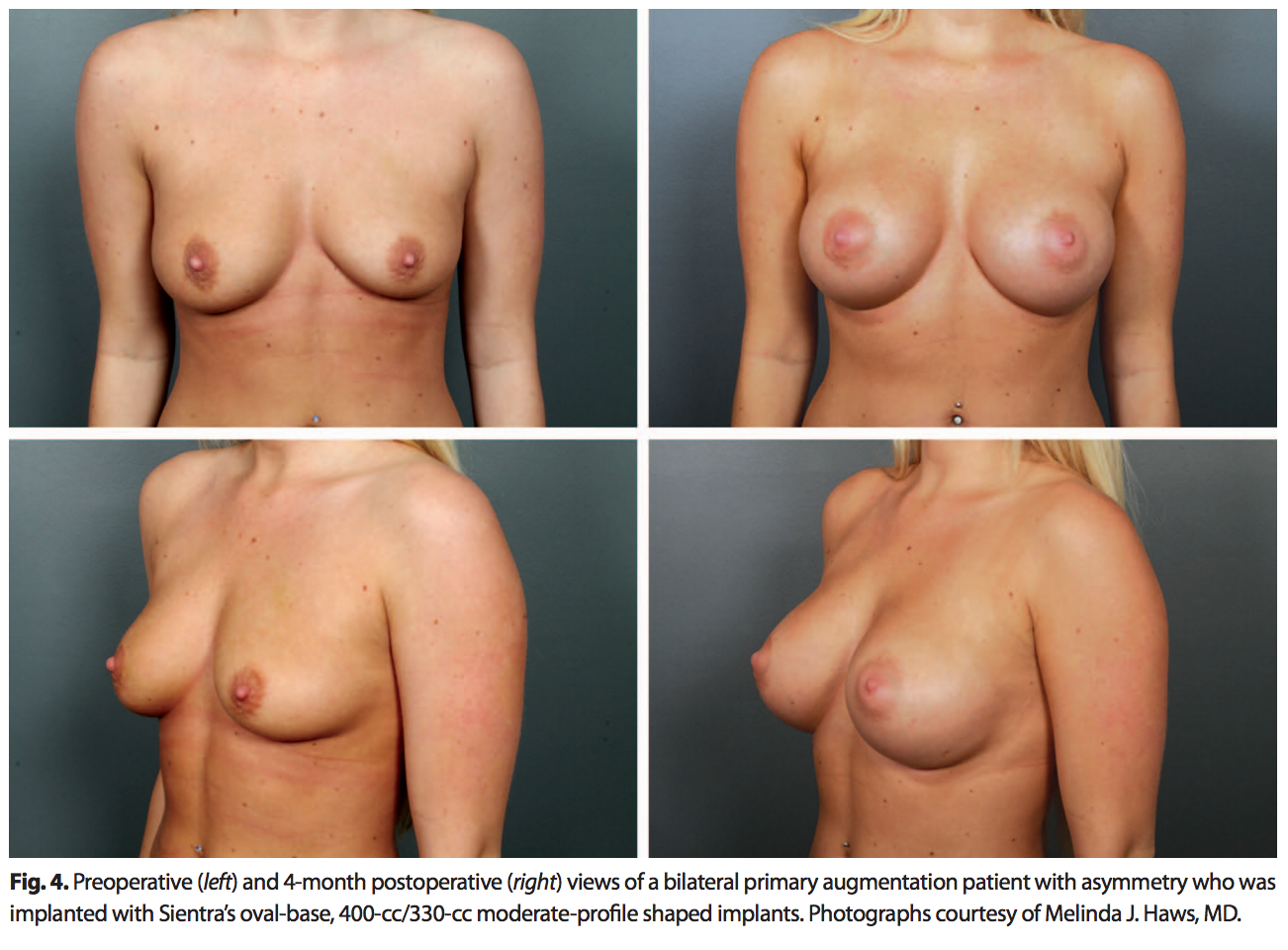
The oval base is appropriate for women who want more volume without compromising a softer, more natural upper pole. A patient population that can benefit from an oval-base implant is women with a wide chest wall and wide breast base (Fig. 4).The oval base is also helpful in patients with axillary fullness to prevent pushing more tissue into this area.
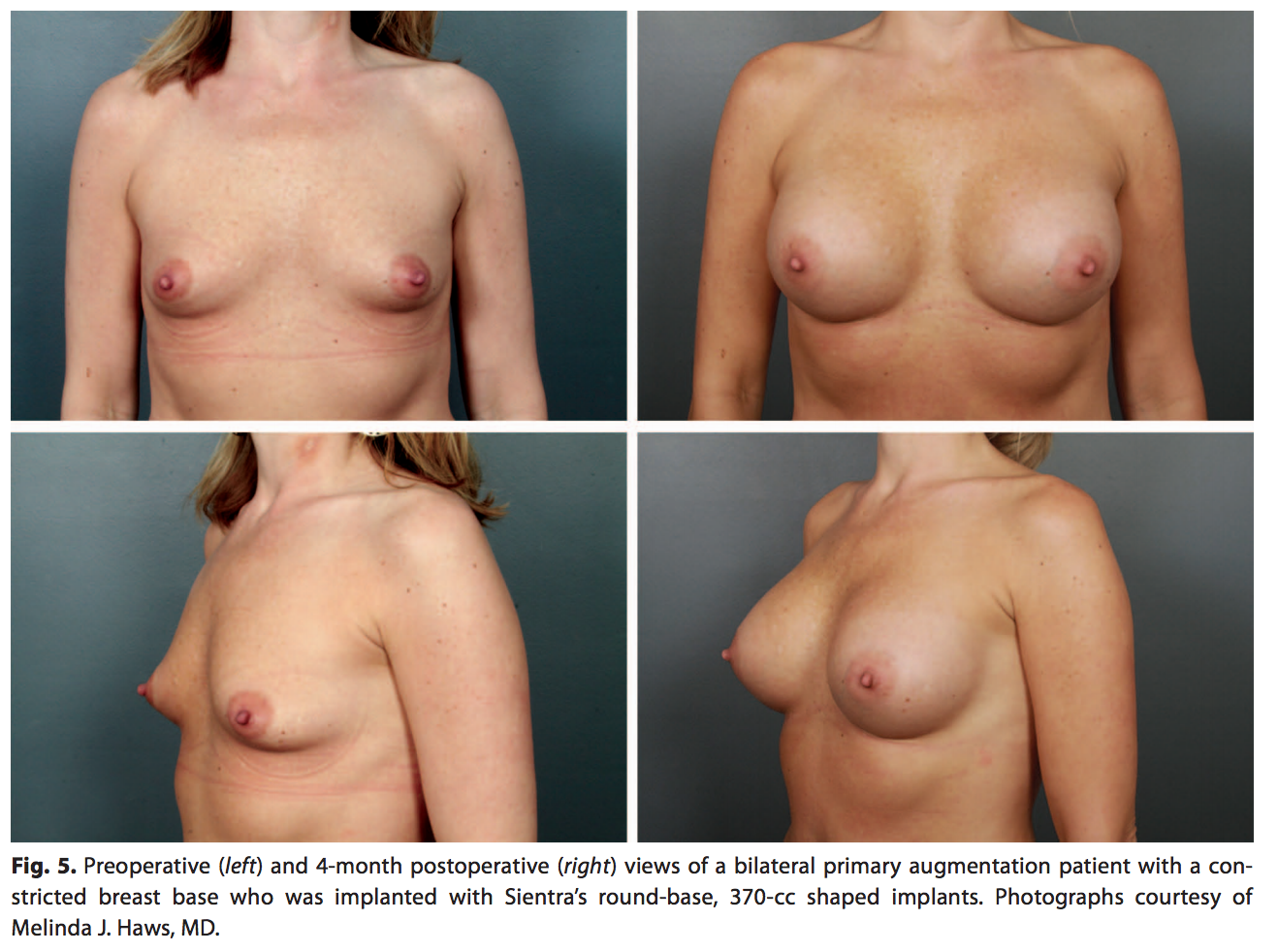
The round-base shaped implant is appropriate for most women desiring a softer upper-pole transition while heightening the position of the nipple-areola complex and expanding the lower pole (Fig. 5). This shape is helpful in women with a tight lower pole or constricted breast base and women with an average breast width and shape who want a natural augmentation.
The classic-base shaped implant, slightly taller than it is wide, is appropriate for women who have a long chest or low inframammary folds. This classic-base shaped implant prevents the emptiness just above the breast that can be observed in these “long-chested” patients.
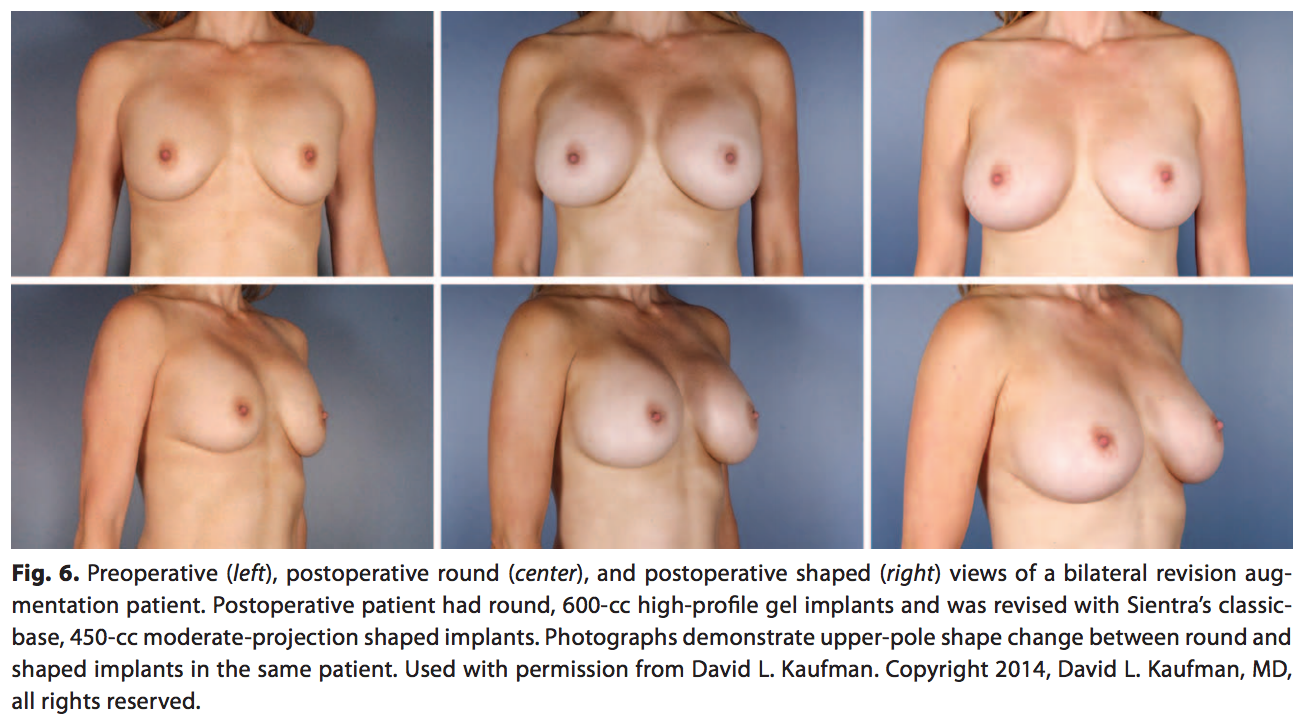
Although both round implants and the shaped implants provide aesthetically pleasing and safe surgical outcomes, they provide 2 distinct outcomes in shaping the breast. The difference can be noted in the more natural, upper-pole slope with the shaped implant compared with the more rounded upper pole of the round implant (Fig. 6). This figure depicts a primary augmentation patient with no complications but dissatisfaction with the roundness in the upper pole and the desire for a more natural shape to her breasts. The patient previously had 600-cc high-profile, smooth, round implants that were replaced with Sientra’s classic-base shaped 450-cc, moderate-profile, textured implants to provide the preferred natural look. Integrating shaped implants into a surgeon’s implant selection provides a wider range of surgical options to individually match patients’ desires.
In addition to the desire for a more natural upper-pole slope, patients also present requesting a firm, youthful breast and can benefit from the HSC+ implant’s slightly firmer gel that provides shape and structure, thereby returning the breast to a more youthful appearance. In the case where a patient seeks a very soft outcome, a round gel may better provide that result.